Available to mentor

The Giessen Lab is interested in the structure, function, and engineering of protein organelles, protein machines, and enzyme filaments. Our current focus lies on microbial protein organelles, compartments, and filaments involved in detoxification, nutrient utilization, and natural product biosynthesis, as well as on the discovery and characterization of novel enzyme machines and assemblies involved in the biosynthesis of bioactive compounds. We endeavor to leverage our molecular level insights into large protein assemblies for synthetic biology applications in drug delivery, biocatalysis, and bionanotechnology. Our interdisciplinary work utilizes techniques and approaches spanning the fields of biochemistry, structural biology (cryo-EM and x-ray crystallography), microbiology and synthetic biology.
Giessen Lab Website Tobias W. Giessen - Google Scholar
-
Postdoctoral Research FellowHarvard Medical School | Wyss Institute for Biologically Inspired Engineering at Harvard, Systems Biology, 2018
-
Postdoctoral Research FellowLoewe Center for Synthetic Microbiology, Marburg, 2014
-
PhD in BiochemistryPhilipps-University Marburg, Marburg, 2013
-
Diplom (BS/MS) in ChemistryPhilipps-University Marburg, Marburg, 2010
-
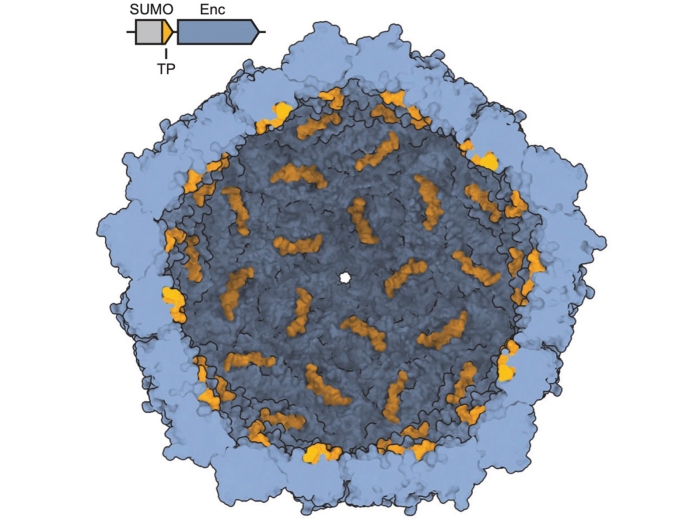
Giessen TW. Chembiochem, 2024 Sep 27; e202400535Journal ArticleThe Structural Diversity of Encapsulin Protein Shells.
DOI:10.1002/cbic.202400535 PMID: 39330624 -
Kwon S, Andreas M, Giessen T. ACS Nano, 2024 Sep 3;Journal ArticlePore Engineering as a General Strategy to Improve Protein-Based Enzyme Nanoreactor Performance
DOI:10.1021/acsnano.4c08186 -
Benisch R, Giessen TW. Protein Sci, 2024 Aug; 33 (8): e5129Journal ArticleStructural and biochemical characterization of an encapsulin-associated rhodanese from Acinetobacter baumannii.
DOI:10.1002/pro.5129 PMID: 39073218 -
Szyszka TN, Andreas MP, Lie F, Miller LM, Adamson LSR, Fatehi F, Twarock R, Draper BE, Jarrold MF, Giessen TW, Lau YH. Proc Natl Acad Sci U S A, 2024 May 14; 121 (20): e2321260121Journal ArticlePoint mutation in a virus-like capsid drives symmetry reduction to form tetrahedral cages.
DOI:10.1073/pnas.2321260121 PMID: 38722807 -
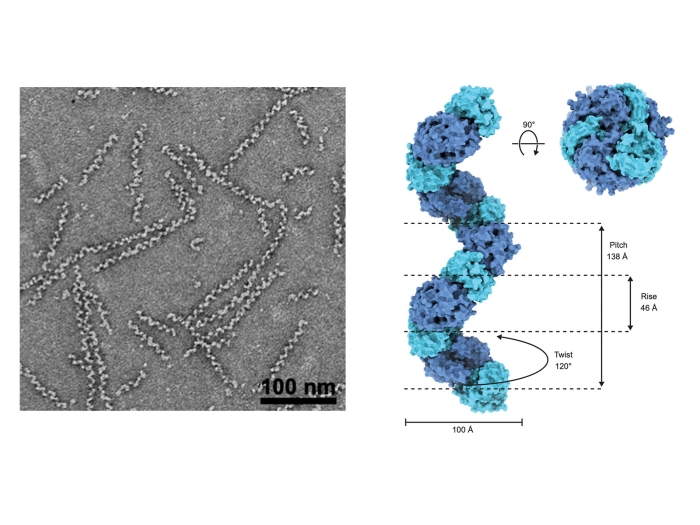
Andreas MP, Giessen TW. Nat Commun, 2024 Apr 27; 15 (1): 3574Journal ArticleCyclodipeptide oxidase is an enzyme filament.
DOI:10.1038/s41467-024-48030-9 PMID: 38678027 -
Dutcher CA, Andreas MP, Giessen TW. bioRxiv, 2024 Apr 26;Journal ArticleA two-component quasi-icosahedral protein nanocompartment with variable shell composition and irregular tiling.
DOI:10.1101/2024.04.25.591138 PMID: 38712103 -
Andreas M, Giessen TW. bioRxiv, 2024 Apr 23;Journal ArticleThe biosynthesis of the odorant 2-methylisoborneol is compartmentalized inside a protein shell
-
Andreas M, Giessen TW. bioRxiv, 2024 Apr 23;Journal ArticleThe biosynthesis of the odorant 2-methylisoborneol is compartmentalized inside a protein shell